Amino Acids
Oct 05 2015
Proline (pro,P) - α helix breaker, rigid, secondary amino group
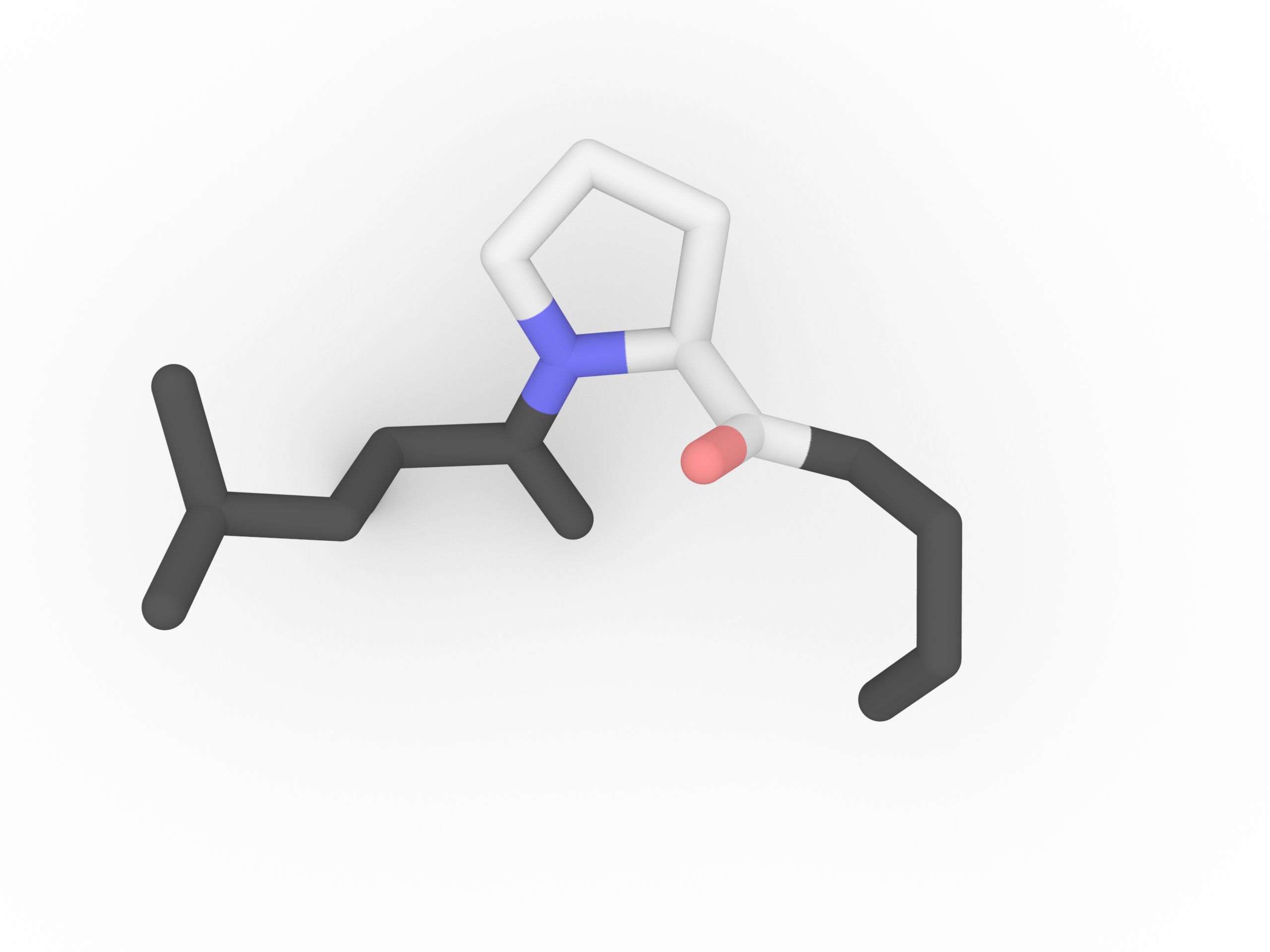
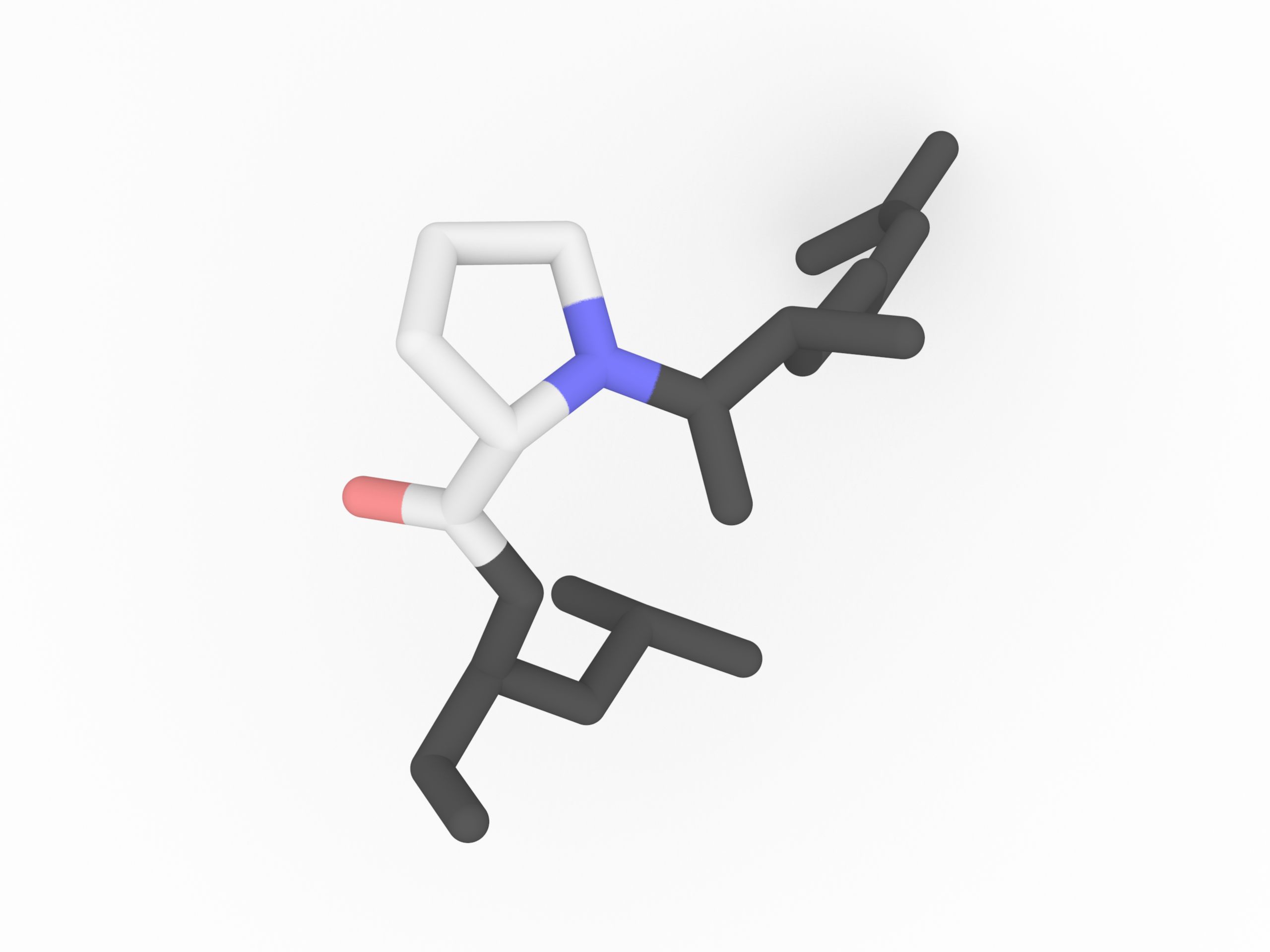
 When Proline is in a peptide bond, it does not have a hydrogen on the α amino group, so it cannot donate a hydrogen bond to stabilize an α helix or a β sheet. When proline is found in an α helix, the helix will have a slight bend due to the lack of the hydrogen bond. Proline is often found at the end of α helix or in turns or loops.
When Proline is in a peptide bond, it does not have a hydrogen on the α amino group, so it cannot donate a hydrogen bond to stabilize an α helix or a β sheet. When proline is found in an α helix, the helix will have a slight bend due to the lack of the hydrogen bond. Proline is often found at the end of α helix or in turns or loops.

Protein secondary structure can be described in terms of the dihedral angles φ, ψ and ω of the protein backbone. The cyclic structure of proline's side chain locks the angle φ at approximately −60°.
Glycine (gly,G) - α helix breaker, flexible H
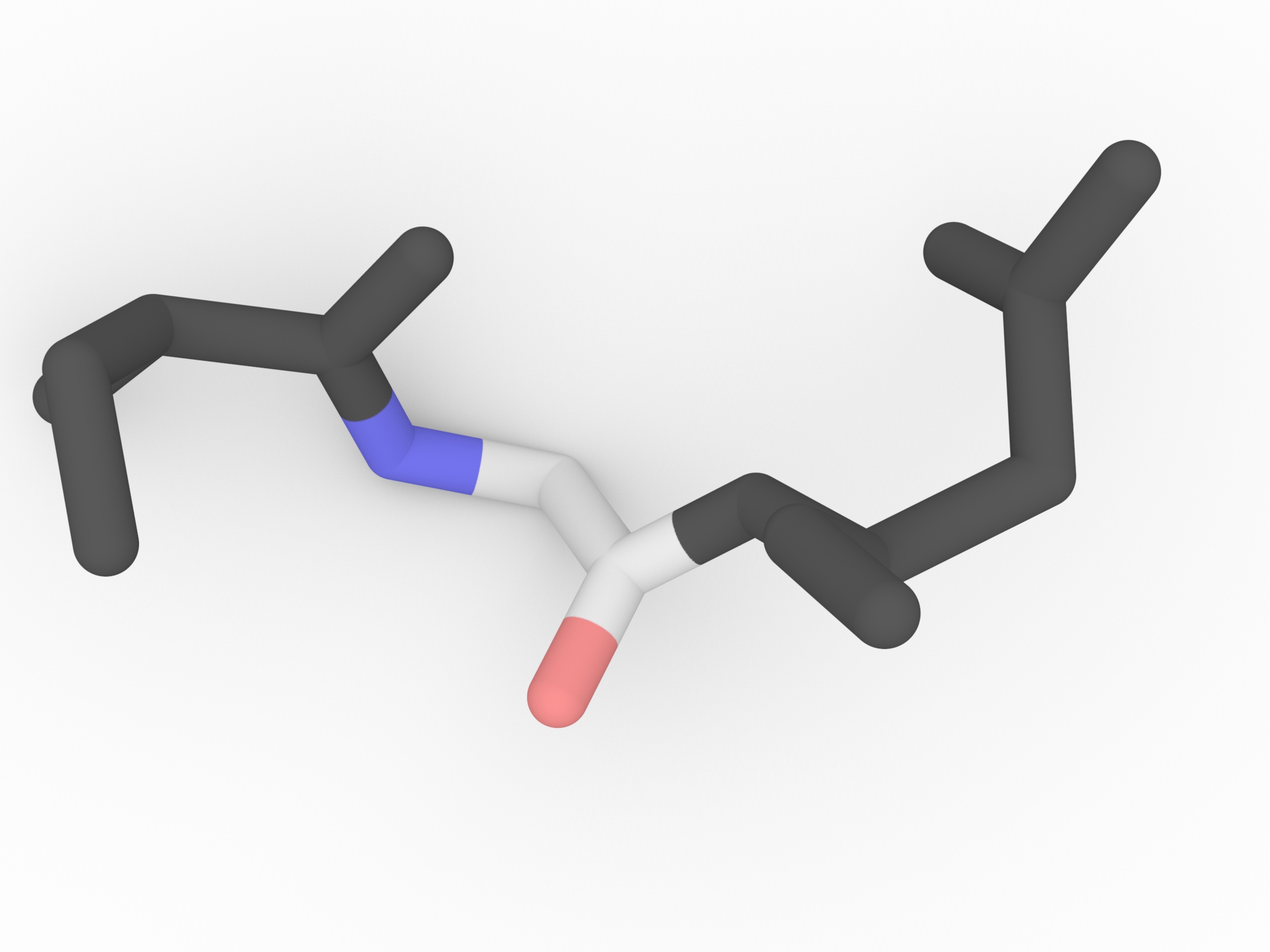
 Glycine is the smallest amino acid. There is no chiral α carbon in Glycine since the side chain is a hydrogen atom. It also has high flexibility due to this single H side chain. Glycine and Proline both can disrupting α helix.
Glycine is the smallest amino acid. There is no chiral α carbon in Glycine since the side chain is a hydrogen atom. It also has high flexibility due to this single H side chain. Glycine and Proline both can disrupting α helix.

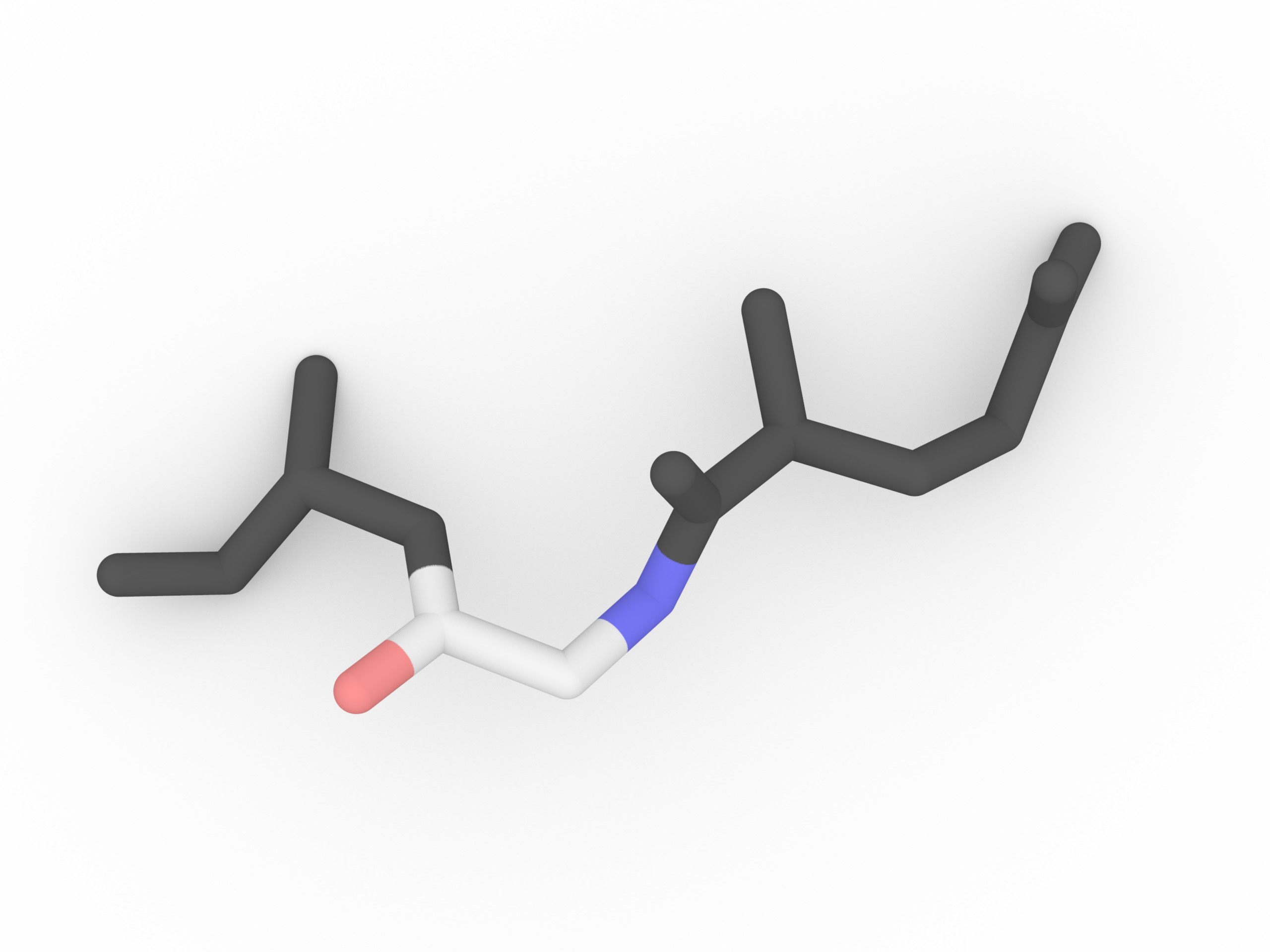
Cysteine (cys,C) - disulfide bond
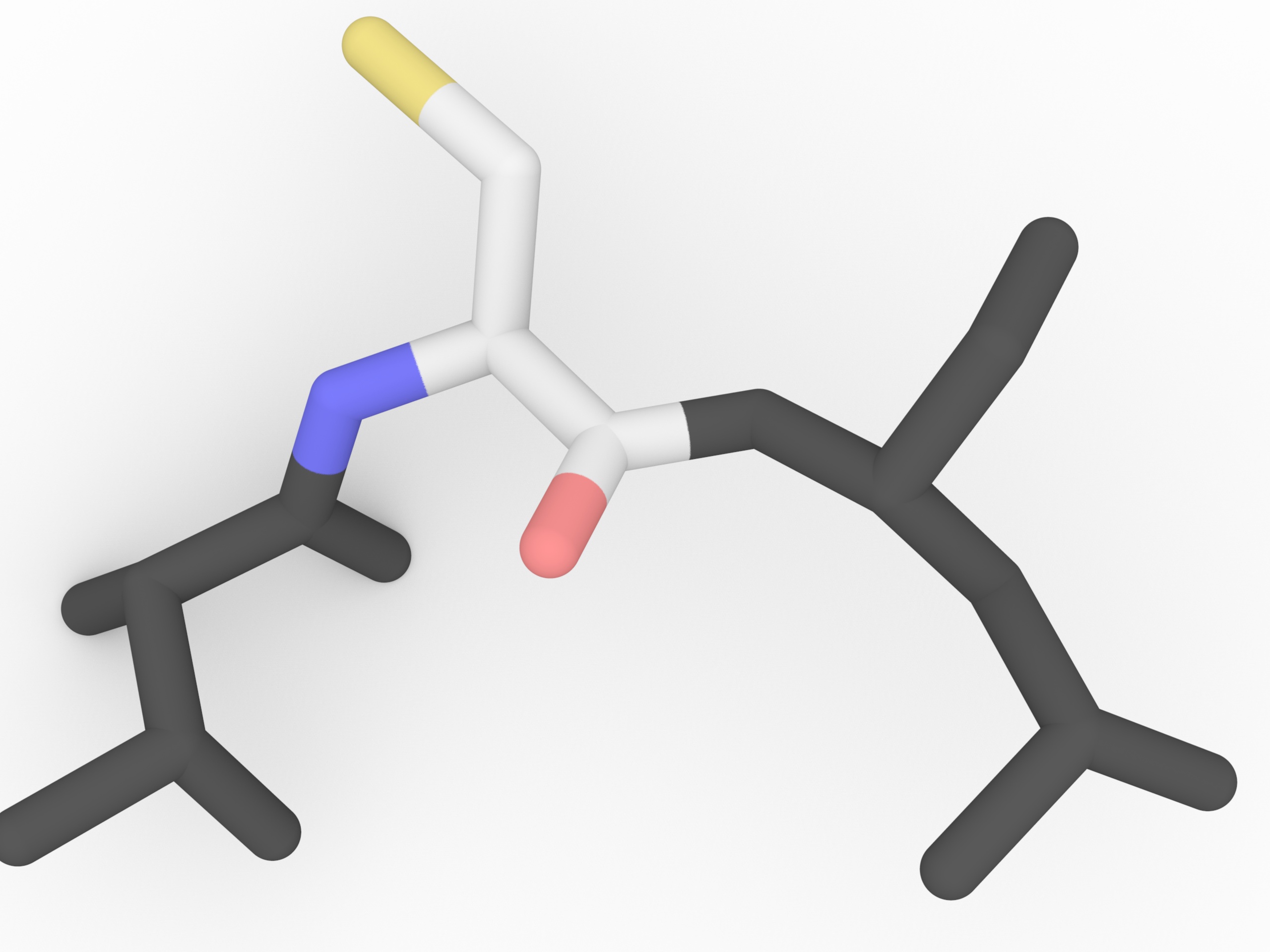
 Cysteine Cysteine as a thiol group (-SH) in the side chain, which can form a disulfide bond (bridge) with another closed cysteine. In the disulfide bond both -SH groups lose the H and the bridge is formed between two S.
Cysteine Cysteine as a thiol group (-SH) in the side chain, which can form a disulfide bond (bridge) with another closed cysteine. In the disulfide bond both -SH groups lose the H and the bridge is formed between two S.

In all organisms studied except plants, two cysteines are frequently found two amino acid residues apart (C-(X)2-C motif). Such a motif is known to be present in a variety of metal-binding proteins and oxidoreductases.
In a statistical analysis of the frequency with which amino acids appear in different chemical environments in the structures of proteins, free cysteine residues were found to associate with hydrophobic regions of proteins. Their hydrophobic tendency was equivalent to that of known non-polar amino acids such as methionine and tyrosine (tyrosine is polar aromatic but also hydrophobic), and was much greater than that of known polar amino acids such as serine and threonine.
References:
Miseta, Attila, and Peter Csutora. "Relationship between the occurrence of cysteine in proteins and the complexity of organisms." Molecular biology and evolution 17.8 (2000): 1232-1239.
Nagano N, Ota M, Nishikawa K (September 1999). "Strong hydrophobic nature of cysteine residues in proteins". FEBS Lett. 458 (1): 69–71. doi:10.1016/S0014-5793(99)01122-9.
Histidine (his,H) - both protonated & deprotonated form
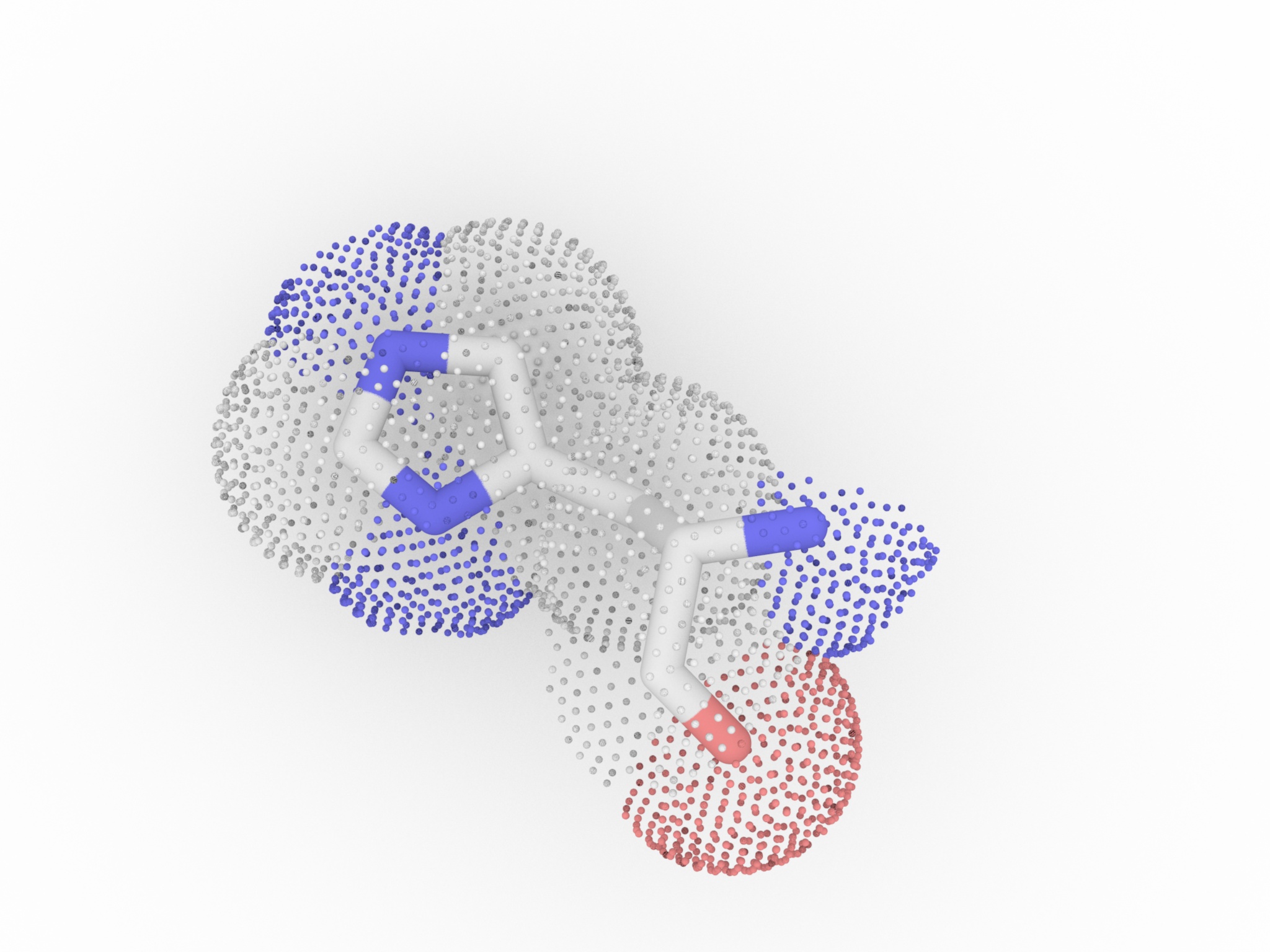
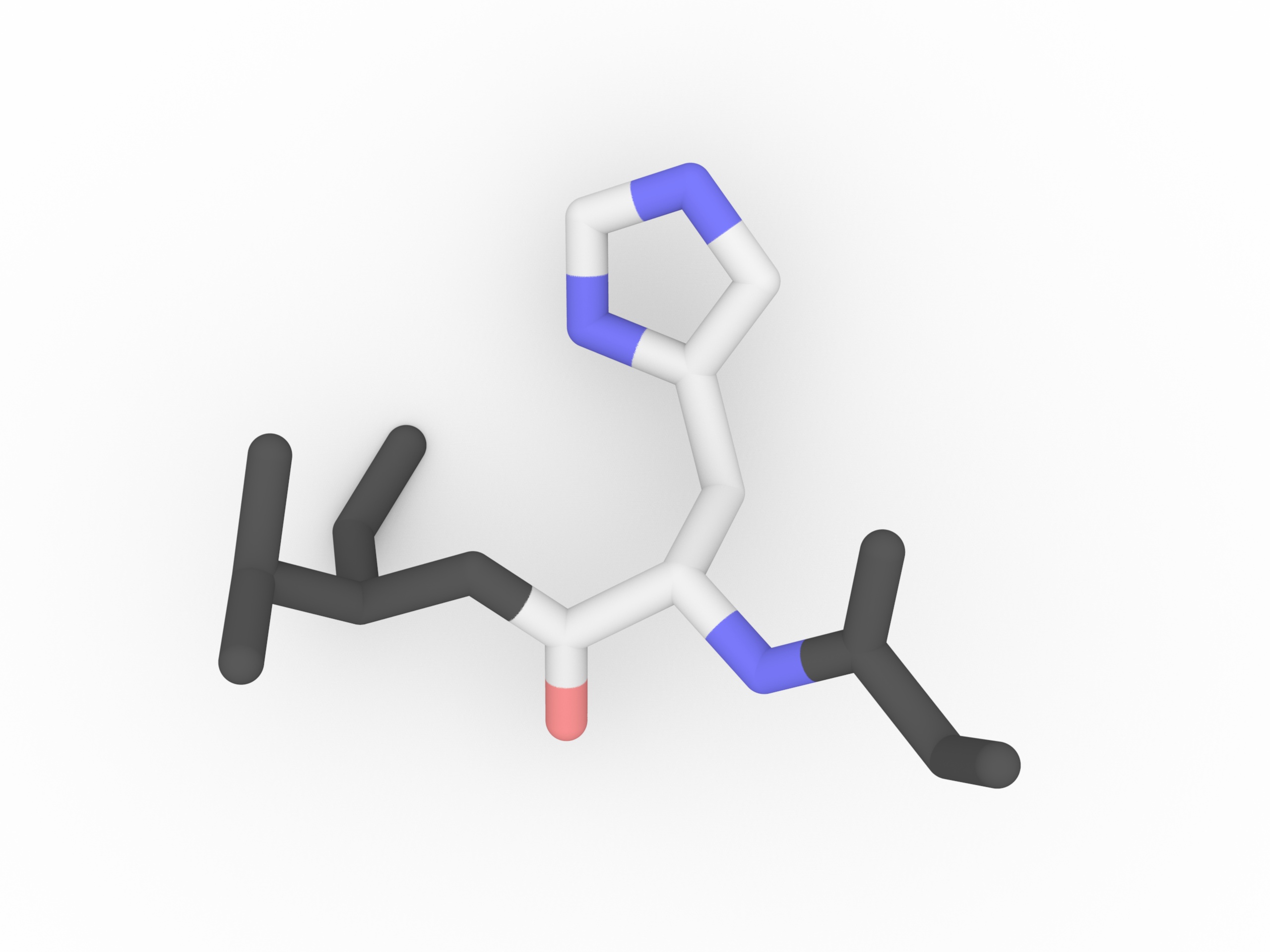
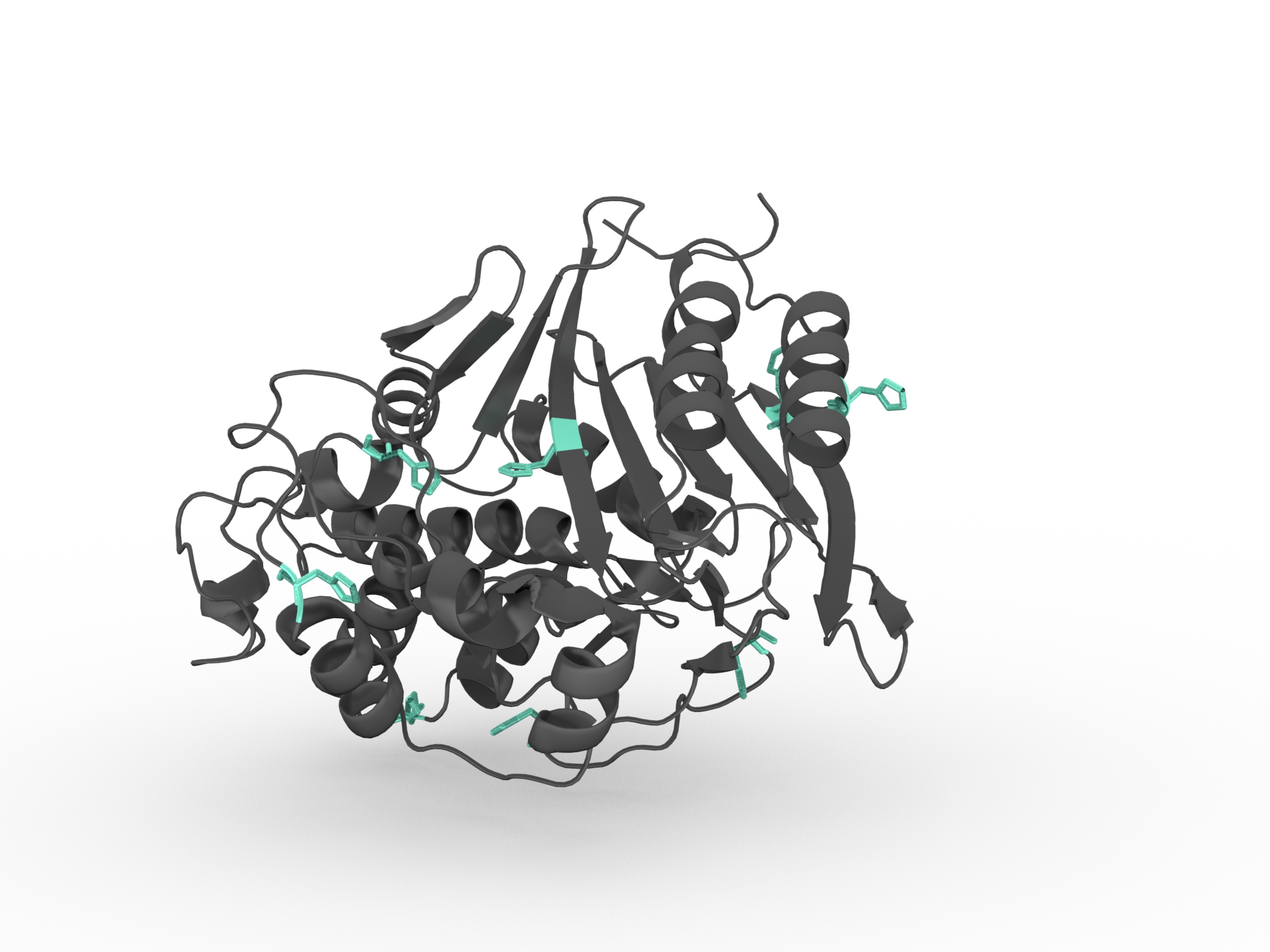 Histidine has a side chain with pKa = 6.5. It is very close to the physiological pH (7). Then Histidine is going to exist in both protonated and deprotonated form. It is particularly useful when it is in the active site of a protein
, where it can both stablize or disablize a substrate. Histidines is rather ambiguous about whether it prefers to be buried in the protein core, or exposed to solvent.
Histidine has a side chain with pKa = 6.5. It is very close to the physiological pH (7). Then Histidine is going to exist in both protonated and deprotonated form. It is particularly useful when it is in the active site of a protein
, where it can both stablize or disablize a substrate. Histidines is rather ambiguous about whether it prefers to be buried in the protein core, or exposed to solvent.

They are very common in metal binding sites (e.g. zinc), often acting together with Cysteines or other amino acids. Thus in this context, it is common to see Histidine replaced by Cysteine.
References:
M.J. Betts, R.B. Russell. Amino acid properties and consequences of subsitutions.
In Bioinformatics for Geneticists, M.R. Barnes, I.C. Gray eds, Wiley, 2003